Specimen Collection
Introduction
Proper sample collection and handling is an integral
part of obtaining a valid and timely laboratory test result. Specimens must be
obtained using proper phlebotomy techniques, collected in the proper container,
correctly labeled (in the presence of the patient) and promptly transported to
the laboratory. It is the policy of the laboratory to reject samples when
there is failure to follow these guidelines. All specimens should be
handled with universal precautions, as if they are hazardous and infectious.
Patient Preparation
Prior to each collection, review the appropriate
test description, including the specimen type to be collected, the minimum volume required, the
procedure, the collection materials, and the storage and handling instructions.
Specimen Collection Timing
The basal state (the early morning approximately 12
hours after the last ingestion of food) is recommended for determining the
concentration of body constituents such as glucose, cholesterol, triglycerides,
electrolytes, and proteins.
Blood composition is significantly altered after
consuming food, and consequently alters many clinical chemistry tests. For
outpatients, provide the patient in advance with appropriate collection
instructions and information on fasting, diet, and medication restrictions when
necessary.
Universal Precautions
All specimens should be regarded as potentially
hazardous or infectious. Universal Blood and Body Fluid precautions should
be observed.
Test Request Criteria
The test requisition (paper or electronic) must include the following. Hand written requisitions must be legible.
- the patient's first and last name
- a unique identification number which assures positive patient identification (e.g., UH number)
- patient sex
- the unit/clinic location
- the name(s) and signature of the authorized provider requesting the test(s)
- UTMB physician identification number (if applicable)
- the test(s)/procedure(s) being ordered
- the date (including day, month, and year) and time of specimen collection is required on all orders (paper or electronic) or on the actual specimen container
- name or initials of person collecting specimen is required on all orders (paper or electronic) or on the actual specimen container
- pager/ phone number of appropriate contact person
- office address of requesting physician if not part of the UTMB enterprise
- diagnosis: ICD10 code or narrative description
- source of the specimen, when appropriate
- date of last menstrual cycle (for gynecologic specimens)
- clinical Information, when appropriate
- blood bank request forms must include the identity of the phlebotomist
Labeling Criteria
All specimens submitted to
Laboratory Services for testing must be appropriately labeled to assure positive identification and optimum
integrity of patient specimens from the time of collection until testing is
completed and the result reported. In accordance with standards
issued by the College of American Pathologists (CAP), American Association of
Blood Banks, and The Joint Commission, all specimens
must be labeled at the time of collection; in the presence of the patient,
to maintain identity throughout the pre-analytical, analytical, and
post-analytical processes. Refer to
LS I.1.02.01 Specimen Labeling and Requests for Testing for additional requirements.
Orders (paper or electronic) will be checked carefully upon receipt in the laboratory and must match the specimen to be acceptable for testing.
All primary specimen containers must be labeled with two identifiers at the time of collection. If a second patient identifier used is not listed below, then approval to process the specimen must be obtained from the division Medical Director (not applicable to Blood Bank).
- UTMB Specimens (Non-Blood Bank) must be labeled with the patient’s full first and last name and at least one of the following additional unique identifiers:
- Patient’s medical record number
- Offender patient ID number
- UNOS number
- Date of birth
- Sample ID (accession number)
- Blood Bank Specimens must be labeled with both of the following unique identifiers:
- Patient's full name (first and last) and
- Medical record number.
NOTE: Alternative identifiers are NOT accepted for blood bank specimens.
- Non-UTMB Specimens may have patient’s first and last name and one other unique identifier. Acceptable unique identifiers are:
- Date of Birth
- UNOS
- TDCJ Offender ID
- Outside Consults may be accepted with patient’s first and last name, one additional patient identifier (date of birth, sample ID, medical record number) and a copy of the report from the referring hospital.
- All secondary specimen containers (including slides, blocks, and aliquots) are labeled with the patient’s first and last name and at least one of the following additional unique identifiers:
- Patient’s medical record number
- Offender patient ID number
- UNOS number
- Date of birth
- Sample ID (accession number)
Aliquots are also labeled with the specimen type.
Specimen Collection
Prioritize Collection:
"STAT" means special turnaround time and must be collected immediately. It may involve a patient whose medical condition has suddenly become very critical and must be treated as a medical emergency.
Fasting: Requests for "fasting" specimens are performed before routine requests, so patients can
eat meals on schedule.
Order of Draw
Blood cultures, using the appropriate aseptic technique, are always collected first. Blood collection tubes must be drawn in a specific
order to avoid cross-contamination of additives between tubes. The recommended order of draw for is demonstrated below: (click image for larger view)
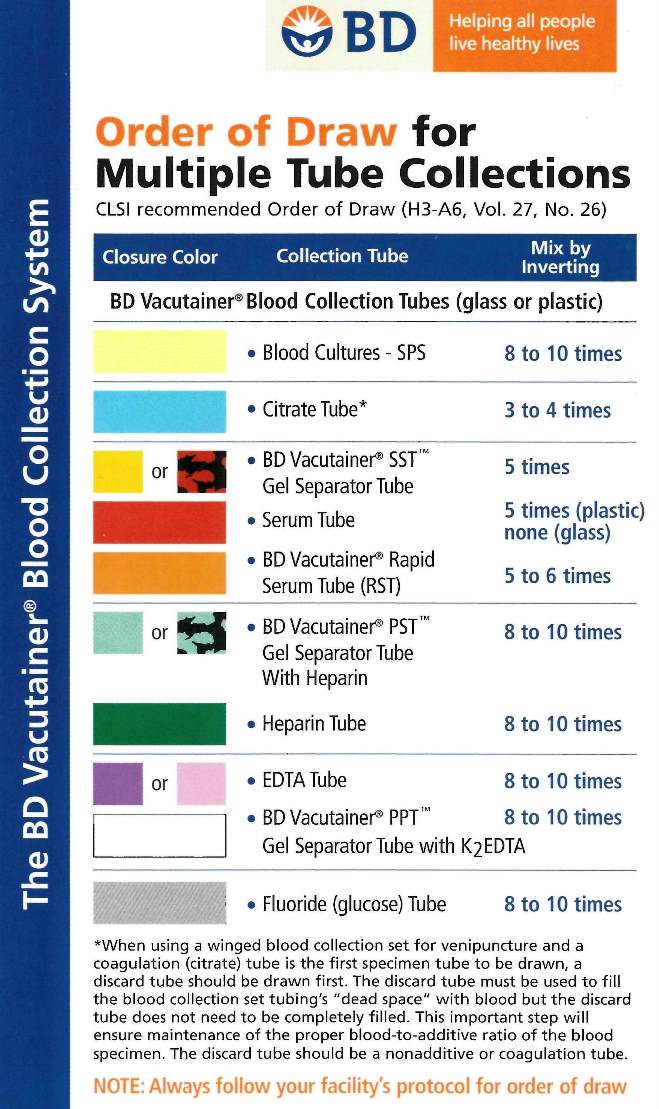
Note: Tubes with additives must be
filled to their stated volumes and thoroughly mixed. Erroneous test results may be obtained when the tube is not filled to the stated volume and blood is not thoroughly mixed with the additive. When using a winged blood collection set for venipuncture and a coagulation (citrate) or lavender (EDTA) tube is the first specimen to be drawn, a discard tube should be drawn first. The discard tube (a non-additive or coagulation tube) must be used to displace the air in the collection set’s tubing, but does not need to be filled completely. Mix all tubes with anticoagulant by gentle inversion according to the chart above.
Urine Specimen Collection
Random Urine Collection
First voided morning specimen
s should be collected in clean containers and transported to the laboratory within 30 minutes or stored according to the specific instructions provided for the test requested.
Timed Urine Collection
- Containers (brown plastic bottles) for timed urine collections are provided by Sample Management, room CSW 7.412.
- Appropriate preservative is added to the container by the laboratory personnel when necessary. Refer to the alphabetical listing of tests for collection instructions for the required laboratory test.
- Appropriate precautionary labels are affixed to the collection bottles containing hazardous chemicals. Read and follow the precautions.
- Specimens requiring refrigeration may be placed in a basin filled with crushed ice if a refrigerator is not available.
To ensure accurate results, it is essential that an accurate collection of the timed output of urine is achieved. Follow these guidelines to collect a timed urine:
- At the beginning of the collection period, the bladder should be emptied.
- Discard this sample and note the time on the requisition form.
- Collect all urine passed from this point on.
- Specimens are placed in the timed urine bottle which may contain preservatives. Use caution when adding the urine to bottles containing acid preservatives. For safety purposes, instruct the patient to urinate in a clean, dry container, then transfer the urine to the collection bottle. The patient should not urinate into the collection bottle.
- If one of the samples is accidentally lost or removed within the collection period, the entire collection must be restarted.
- At the end of the collection period the bladder is emptied and the specimen is added to the collection.
- Note the ending time on the request form.
- If removing an aliquot of urine, the total volume for the timed collection must be noted on the request form.
Clean Catch, Mid-Stream Urine collection:
Females
- Remove appropriate garments for urination.
- Wash hands thoroughly.
- With one hand, separate the urinary opening and hold open until the process is complete.
- Using a saturated soapy sponge, wash from front to back three times using a different soap and pad each time.
- Wash with warm sterile water to remove soap residue.
- The patient should void a few milliliters into the toilet and then collect urine in a sterile container. (Do not stop the flow of urine through this process.)
- Secure the lid on the container.
- The nurse will transfer the specimen for culture to the gray-topped vacutainer tube through needle apparatus on urine cup. Refer to package insert for further information/instructions.
Males
- Remove appropriate garments for urination
- Wash hands thoroughly with soap and water.
- Cleanse the penis, retract the foreskin (if not circumcised), and wash three times with a different soap pad each time.
- Wash with warm sterile water to remove the soap residue.
- Keeping the foreskin retracted, allow a few milliliters of urine to pass and then collect urine in a sterile container. (Do not stop the flow of urine through this process.
- Secure the lid on the container.
- The nurse will transfer the specimen for culture to the gray-topped vacutainer tube through needle apparatus on urine cup. Refer to package insert for further information/instructions.